[from Development to release]
Confidence In Every Molecule
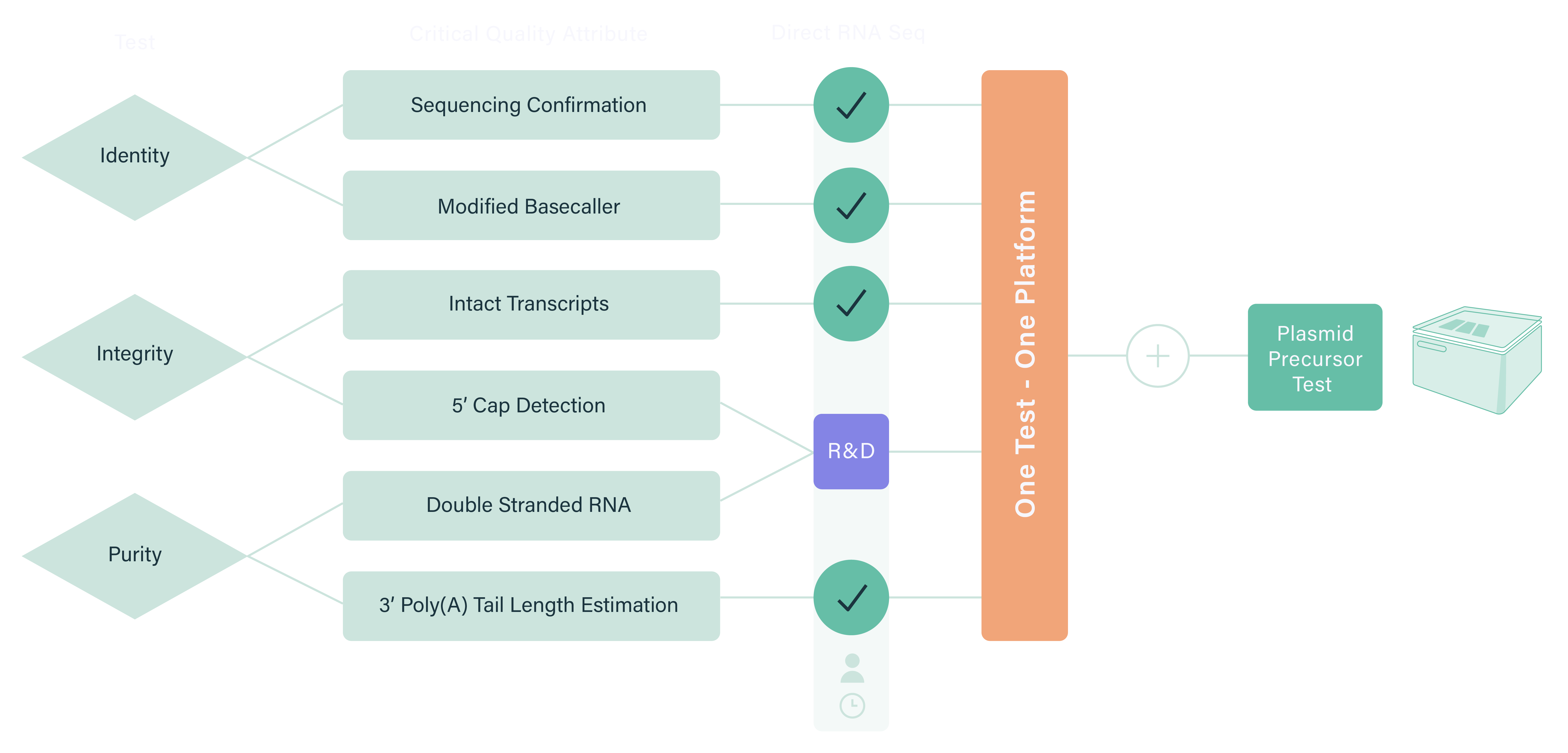
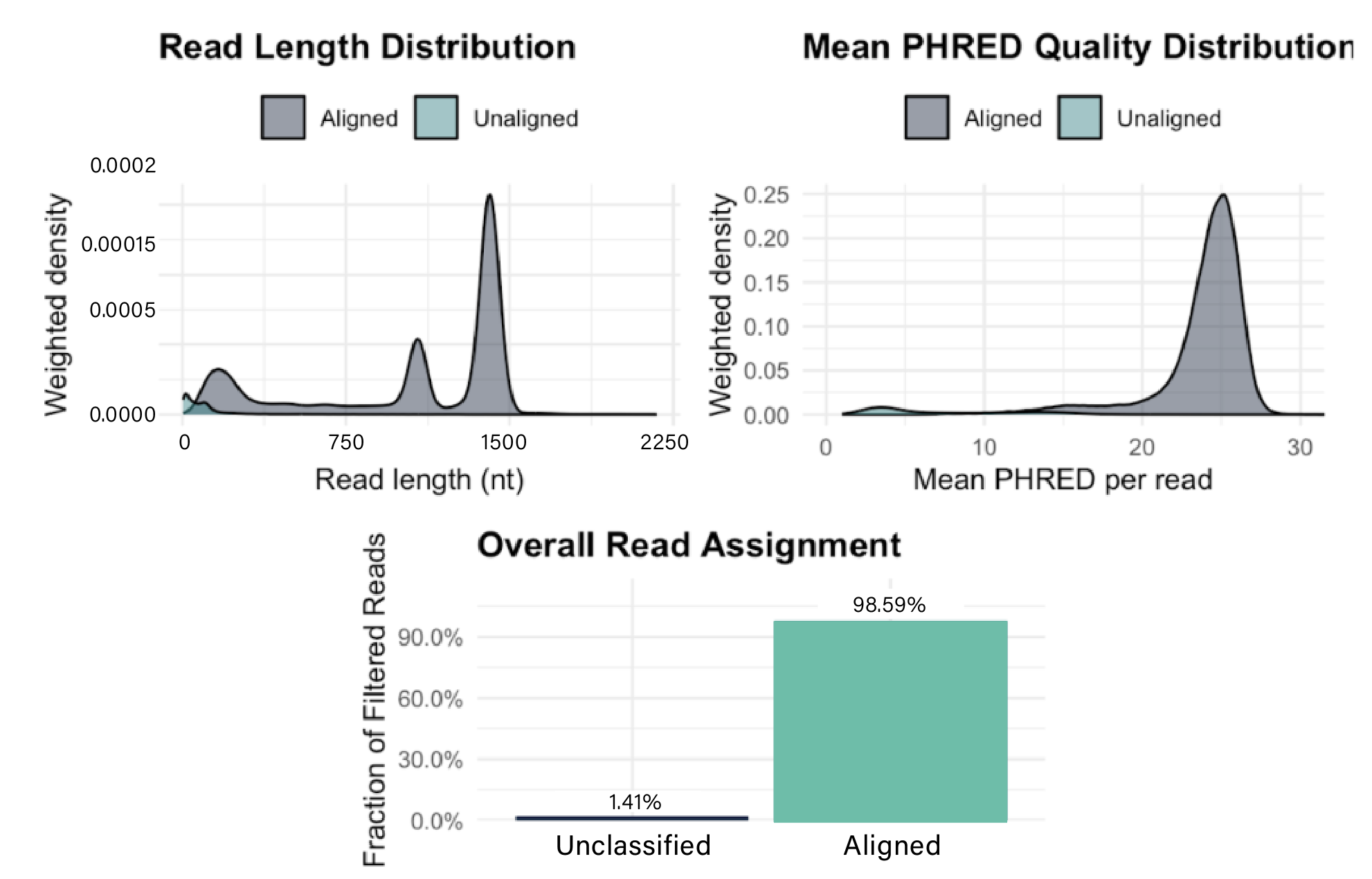
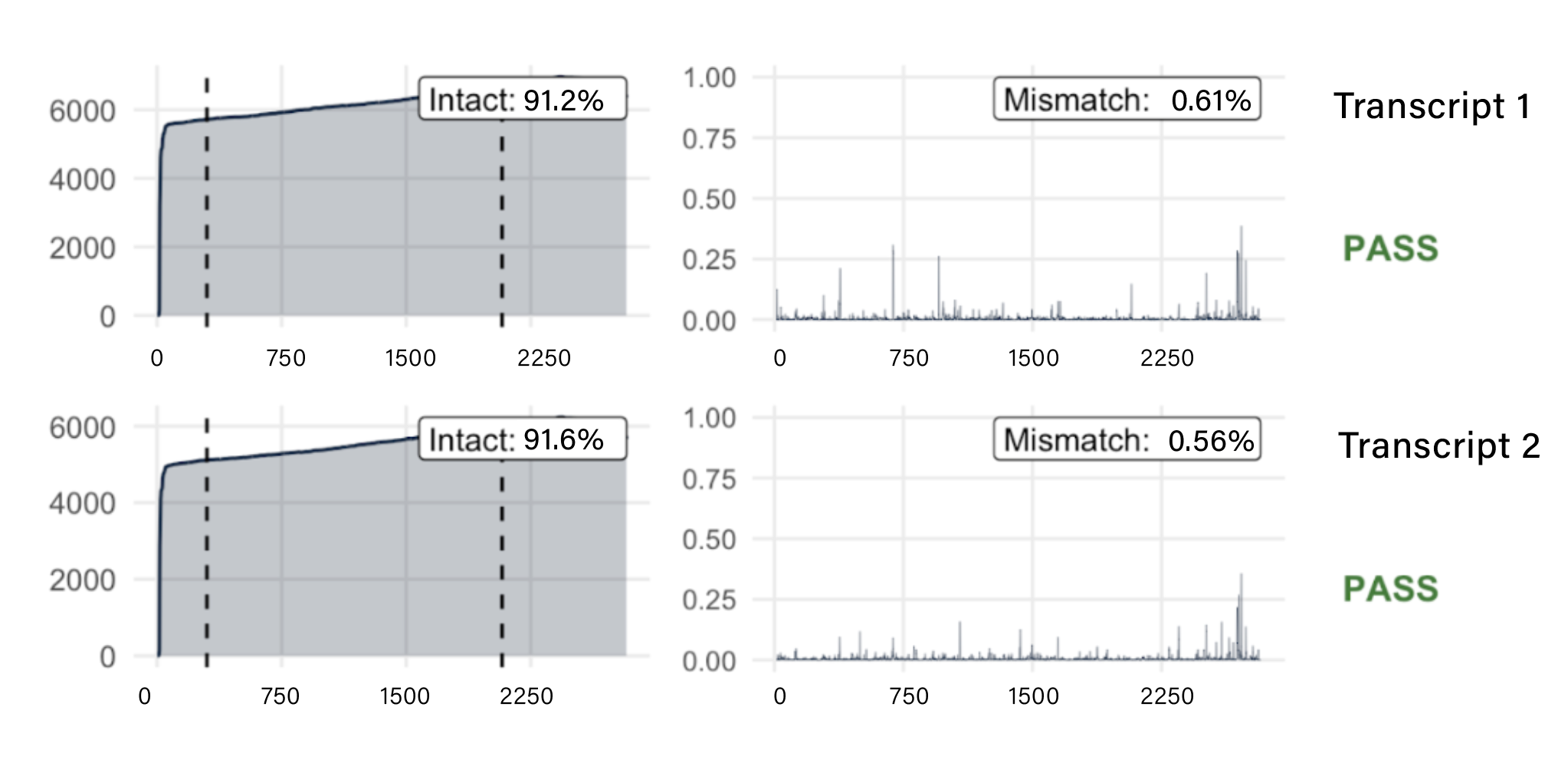
Wasatch BioLabs’ Oxford Nanopore mRNA Vaccine QC service provides complete construct validation in a single assay. Using direct RNA sequencing, we capture full-length mRNA molecules without amplification or reverse transcription, preserving 5′/3′ integrity, poly(A) tail length, and native sequence fidelity. Whether you’re developing novel mRNA constructs or performing lot-release testing, our workflow delivers single-molecule resolution and comprehensive insight across every transcript.